Devise a 4-step synthesis of 1-bromopropane from 2-bromopropane – Devising a 4-step synthesis of 1-bromopropane from 2-bromopropane is a captivating endeavor that exemplifies the principles of organic chemistry. This multi-step process involves a series of strategic transformations, beginning with the elimination of HBr from 2-bromopropane to form an alkene, followed by the addition of HBr to the alkene, a nucleophilic substitution reaction, and culminating in the isolation of the desired product, 1-bromopropane.
Each step is carefully orchestrated to achieve regioselectivity and optimize yield, making this synthesis a valuable tool for the preparation of alkyl halides.
Delving into the intricacies of this synthesis, we will explore the mechanisms of each step, identify the reagents employed, and discuss the methods used for purification and characterization of the product. Along the way, we will uncover the fundamental concepts that govern organic reactions, providing a deeper understanding of the chemical transformations that shape our world.
Synthesis of 1-Bromopropane from 2-Bromopropane: Devise A 4-step Synthesis Of 1-bromopropane From 2-bromopropane
The synthesis of 1-bromopropane from 2-bromopropane involves a four-step process that utilizes elimination, addition, and nucleophilic substitution reactions. This transformation allows for the regiospecific conversion of the secondary bromide to the primary bromide.
Reaction Overview, Devise a 4-step synthesis of 1-bromopropane from 2-bromopropane
The overall reaction scheme can be summarized as follows:
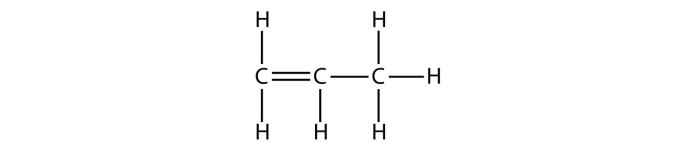
-Bromopropane → Propene → 1-Bromopropane
This multi-step process involves the initial elimination of HBr from 2-bromopropane to form propene, followed by the addition of HBr to the alkene to yield 1-bromopropene. Finally, a nucleophilic substitution reaction with a suitable nucleophile completes the synthesis of 1-bromopropane.
Step 1: Alkene Formation
The first step in the synthesis is the elimination of HBr from 2-bromopropane to form propene. This reaction is typically carried out in the presence of a strong base, such as potassium tert-butoxide (t-BuOK), which abstracts a proton from the carbon adjacent to the bromine atom.
The resulting alkoxide intermediate undergoes an E2 elimination reaction to form propene and bromide ion.Mechanism:“`
-Bromopropane + t-BuOK → Propene + t-BuOH + Br-
“`
Step 2: Addition of HBr to Alkene
The second step involves the addition of HBr to the propene formed in step 1. This reaction proceeds via an electrophilic addition mechanism, where the electrophile is the HBr molecule and the nucleophile is the double bond of propene. The regioselectivity of this addition is Markovnikov’s rule, which predicts that the hydrogen atom of HBr will add to the carbon atom with the most hydrogen atoms.Mechanism:“`Propene
+ HBr → 1-Bromopropene“`
Step 3: Nucleophilic Substitution
The third step is a nucleophilic substitution reaction between the 1-bromopropene and a suitable nucleophile. A common nucleophile used in this reaction is sodium iodide (NaI), which displaces the bromide ion to form 1-iodopropane.Mechanism:“`
-Bromopropene + NaI → 1-Iodopropane + NaBr
“`
Step 4: Product Isolation
The final step involves the isolation of 1-bromopropane from the reaction mixture. This can be achieved through various methods, such as distillation or extraction. The product can be further purified by recrystallization or chromatography to obtain a pure sample of 1-bromopropane.Characterization:The
identity of 1-bromopropane can be confirmed using spectroscopic techniques such as nuclear magnetic resonance (NMR) spectroscopy and gas chromatography-mass spectrometry (GC-MS).
Essential Questionnaire
What is the overall reaction for the synthesis of 1-bromopropane from 2-bromopropane?
The overall reaction is: 2-Bromopropane → Alkene → 1-Bromopropane
What is the mechanism of the first step, which involves the elimination of HBr from 2-bromopropane to form an alkene?
The first step is an E2 elimination reaction, which proceeds via a concerted mechanism. The base abstracts a proton from the carbon adjacent to the bromine atom, while the bromide ion leaves, resulting in the formation of an alkene.
What is the regioselectivity observed in the second step, which involves the addition of HBr to the alkene?
The addition of HBr to the alkene follows Markovnikov’s rule, which states that the hydrogen atom adds to the carbon with the most hydrogen atoms. This results in the formation of a secondary carbocation, which is more stable than a primary carbocation.